Biblioteca Istituto Zooprofilattico Sperimentale dell'Umbria e delle Marche
Sanità Pubblica Veterinaria: Numero 92, Ottobre 2015 [http://www.spvet.it/] ISSN 1592-1581
Documento reperibile all'indirizzo: http://spvet.it/indice-spv.html#613
Approccio biologico alla decontaminazione dei molluschi bivalvi da V. parahaemolyticus tramite Bdellovibrio e organismi similari (BALOs): Una collaborazione tra IZSUM e USDA-ARS
Biological approach to decontamination of bivalve molluscs from V. parahaemolyticus by Bdellovibrio and like-organisms (balos): collaboration between IZSUM and USDA-ARS
Donatella Ottaviani, Serena Chierichetti, Francesca Leoni
Abstract. The conventional approach to the purification is known to have limited effect in reducing Vibrio contamination. Because the majority of pathogenic vibrios occur naturally in coastal and estuarine environments and are not associated with sewage contamination, the relaying of the bivalve molluscs in cleaned marine areas is also not so effective for their natural purification from these microorganisms (Andrews,2004). An innovative one is undoubtedly the biological approach that uses bactericidal activity of certain naturally occurring microorganisms in the marine environment. In this regard, Bdellovibrio-and-like organisms (BALOs), a less well-studied group of ubiquitous bacteria which are also present in the marine environment, are small predatory parasitic bacteria with a unique ability to penetrate, reproduce and subsequently kill other gram-negative bacteria, including
Salmonella and
Vibrio (Ruby, 1991). Moreover, in addition to pathogenic bacteria they are also able to eliminate spoilage bacteria, allowing to obtain a microbiologically and organoleptically improved food. Then, purification with BALOs could be proposed as a post-harvest process for reducing vibrios, other pathogenic and spoilage bacteria present in shellfish, increase their safety and improve food quality and shelf life. The application of biological controls to reduce human pathogens such us vibrios from bivalves has been the subject of interest and study by the laboratory of Food Control of IZSUM Ancona section from as early as 2006. To the light of all these evidences in the year 2012 it was started a collaboration between the team of Donatella Ottaviani and that of Gary P. Richards Lead Scientist of Microbial Safety of Aquaculture Product Center of Excellence United States Department of Agricolture (USDA-ARS) in Dover, Delaware with the common purpose to test the applicability to laboratory scale of BALOs in bivalve molluscs purification from
V. parahaemolyticus, standardizing operative protocols to be applied both on depuration of oysters (team of Gary Richards) and mussels (team of Donatella Ottaviani). Preliminary results show that BALOs are naturally present in water of our collection areas and thet can bioaccumulate in
Mytilus galloprovincialis and inhibit the growth of
V. parahaemolyticus
Riassunto. L'approccio convenzionale alla depurazione dei molluschi bivalvi non è efficace nell'abbattere la contaminazione da Vibrio e quelli innovativi post-raccolta spesso sono fatali per i molluschi bivalvi e non soddisfano quei consumatori che desiderano consumare molluschi crudi. Con la depurazione biologica si potenzia una dinamica che normalmente avviene in natura utilizzando batteri che quotidianamente ingeriamo attraverso alimenti di varia origine. In questa ottica si pongono Bdellovibrio-and-like organisms (BALOs), parassiti intracellulari di Gram-negativi, non patogeni per l'uomo e presenti nel suolo, nelle acque reflue, nell'acqua fresca e nell'acqua di mare. Essendo i BALOs parassiti ad ampio spettro, possono ridurre la carica microbica riferibile a batteria patogeni ed alterativi, permettendo di ottenere contestualmente un alimento microbiologicamente ed organoletticamente migliore. La depurazione con BALOs potrebbe essere proposta come processo d'intervento post-raccolta per ridurre vibrioni, altri patogeni e batteri alterativi presenti nei molluschi per aumentarne la sicurezza e favorirne la qualità e la shelf life. I molluschi bivalvi provenienti dalle zone di raccolta dei nostri mari sono frequentemente contaminati da ceppi tossigeni di V. parahaemolyticus ed eventi gastroenterici dovuti a V. parahaemolyticus legati al consumo di molluschi bivalvi indigeni sono stati frequentemente segnalati in Italia. Per questi motivi da svariati anni il Laboratorio Controllo Alimenti della Sezione di Ancona dell'IZSUM si sta interessando di depurazione biologica da applicare ai molluschi bivalvi, in particolare a Mytilus galloprovincialis. In questa ottica dal 2013 è stata avviata una collaborazione tra il Laboratorio Controllo Alimenti della Sezione di Ancona e the Laboratory of Microbial Safety of Aquaculture Product, Center of Excellence of United States Department of Agricolture (USDA-ARS) in Dover, Delaware con l'intento comune di valutare l'applicabilità dei BALOs alla depurazione dei molluschi bivalvi attraverso la messa a punto e standardizzazione di protocolli operativi da applicare alla eliminazione di V. parahaemolyticus dalle ostriche (Team di Gary Richards, USDA-ARS) e dai mitili (Team di Donatella Ottaviani, IZSUM). I risultati preliminari ottenuti nel Laboratorio di Controllo Alimenti della Sezione di Ancona dell'IZSUM sembrano piuttosto promettenti dimostrando che nelle nostre acque di raccolta dei molluschi bivalvi sono presenti livelli naturali di BALOs e che questi sono in grado di abbattere la carica di V. parahaemolyticus a livello sperimentale attraverso studi di bioaccumulo su Mytilus galloprovincialis
Since the early 1970s, the global consumption of bivalve molluscs has increased considerably with a concomitant rise in the reports of outbreaks of infection (Potasman et al., 2002). Bivalves present a particular risk to the consumer because during the process of filter feeding, pathogens in the environment can be concentrated in the digestive tract. This risk is compounded by traditional practices of eating shellfish either raw or lightly cooked. It is common practice worldwide that bivalve molluscs exceeding the limits of faecal indicators are depurated or relayed in a less contaminated area to reduce microbiological contamination before commercialization (Lee et al.,2008). Moreover, the conventional approach to the purification is known to have limited effect in reducing Vibrio contamination (Barile et al., 2009; Croci et al.,2002).
Because the majority of pathogenic vibrios occur naturally in coastal and estuarine environments and are not associated with sewage contamination, the relaying of the bivalve molluscs in cleaned marine areas is also not so effective for their natural purification from these microorganisms (Andrews,2004). To control V. parahaemolyticus infections, innovative post-harvest treatment of shellfish such as Individual Quick Freezing, HeatCool Pasteurization, and High Hydrostatic Pressure have been developed (Mississipi Department of Marine Resources, USA, 2006).
These technologies, which are currently commercially available, have made possible to bring raw and safer products to consumers. But unfortunately, molluscs cannot survive any of the above technologies and could not satisfy those consumers who ask for live bivalves. Concerns about the safety of mussels consumption prompted to investigate new and effective decontamination systems. An innovative one is undoubtedly the biological approach that uses bactericidal activity of certain naturally occurring microorganisms in the marine environment.
In this regard, Bdellovibrio-and-like organisms (BALOs), a less well-studied group of ubiquitous bacteria which are also present in the marine environment, are small predatory parasitic bacteria with a unique ability to penetrate, reproduce and subsequently kill other gram-negative bacteria, including Salmonella and Vibrio (Ruby, 1991). Since BALOs are non-pathogenic to humans, plants and animals, are currently successfully tested as potential microbial control or therapeutic agents in human medicine, food production, agriculture and aquaculture (Cao et al., 2011; Dashiff et al., 2010; Fratamico and Cooke, 1996; Lu and Cai, 2011; Marozzi et al., 2007). BALOs includes 4 genera: Bdellovibrio, Peredibacter, Bacteriolyticum, Bacteriovorax - marine forms. In the marine environment, BALOs and Bacteriovorax in particular, show high host specificity towards human pathogens, and growth of the pathogens in biofilms or in viable but nonculturable forms does not prevent their predation (Dashiff et al., 2010; Kadouri and OToole, 2005; Markelova N.Y, 2010).
BALOs are more tolerant than other aerobic bacteria to aquatic environmental changes as temperature, salinity, pollution, (Wagner and Loy, 2002). They are also resistant to chemical and physical disinfection treatments commonly used in molluscs depuration plants, as ozone, clore, UV (Wagner and Loy, 2002). Moreover, recent researches report that BALOs may survive sufficient time in the intestine of vertebrates, including bivalves, to have a beneficial therapeutic effect "in vivo" against pathogen bacteria (Atterbury et al., 2011; Li et al., 2011). Different from physical and chemical processing techniques focusing only on post-harvest treatment, BALOs and Bacteriovorax in particular, could control the level of pathogens either in periods of growth of bivalves than at the depuration stage. If effective, this approach could represent the starting point for the development and validation of low-cost alternative decontamination methods of bivalve molluscs from V. parahaemolyticus. In this regard, it has been recently reported the role of BALOs as natural modulators of pathogens in marine environment (Richards et al, 2012) and their application for elimination of V.parahaemolyticus in oysters by bioaccumulation experiments (Li et al 2011).
In Italy interest in the problem related to the natural contamination of mussels by V. parahaemolyticus is growing since a recent work has documented in Mytilus galloprovincialis, the most common native species used to edible scope, a prevalence of toxigenic V. parahaemolyticus higher than reported in other European and extra-European countries (Ottaviani et al., 2010a, 2012a). Moreover, in the last three years several cases of acute gastroenteritis due to V. parahaemolyticus, pandemic and not, with local mussels as the most probable source of infection occurred in central Italy (Ottaviani et al., 2008, 2010b, 2012b). The application of biological controls to reduce human pathogens such us vibrios from bivalves has been the subject of interest and study by the laboratory of Food Control of IZSUM Ancona section from as early as 2006. In fact, a past work involving the team of Donatella Ottaviani demonstrated that non pathogenic bacteria from the marine environment were able to inhibit "in vitro" the growth of V. parahaemolyticus strains isolated from indigenous mussels (Carraturo et al., 2006). In the same period another work which involved the team of Donatella Ottaviani reported the ability of marine BALOs isolated from indigenous samples of water and bivalves to parasitize "in vitro" gram-negative pathogenic bacteria such as vibrios and Salmonella (Marozzi et al, 2007).
To the light of all these evidences in the year 2012 it was started a collaboration between the team of Donatella Ottaviani and that of Gary P. Richards Lead Scientist of Microbial Safety of Aquaculture Product Center of Excellence United States Department of Agricolture (USDA-ARS) in Dover, Delaware with the common purpose to test the applicability to laboratory scale of BALOs in bivalve molluscs purification from V. parahaemolyticus, standardizing operative protocols to be applied both on depuration of oysters (team of Gary Richards) and mussels (team of Donatella Ottaviani). In this context, in March 2014 Serena Chierichetti that is part of the team of Donatella Ottaviani, has completed an internship of 15 days in the laboratory directed by Gary Richards in order to learn the techniques of cultivation and utilization in bioaccumulation experiments of BALOs so that they could be applied at Food Control Laboratory of Ancona (Figure 1).

(a)

(b)
Figure 1a / b. Serena Chierichetti at USDA-ARS Laboratory directed by Gary Richards
We are currently carrying out tests by the standard double layer agar plating technique (Stolp and Starr 1963) to evaluate the natural level of BALOs in sea water in our areas of harvesting of Mytilus galloprovincialis and to identify isolates through analysis of DNA sequencing (Richards et al, 2013) (Figure 2; Table 1).

Figure 2. Standard double layer agar plating technique at USDA-ARS
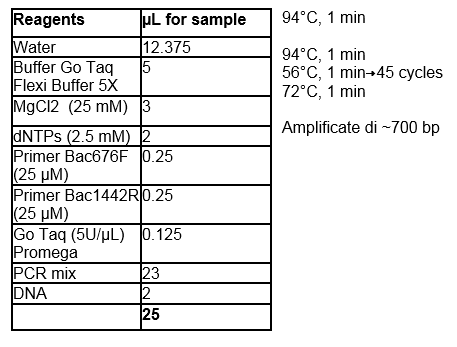
Table 1. PCR protocol for
Bacteriovorax spp identification
Preliminary results show that BALOs are naturally present in water of our collection areas and some of them were belonged to Bacteriovorax spp (Figures 3, 4).
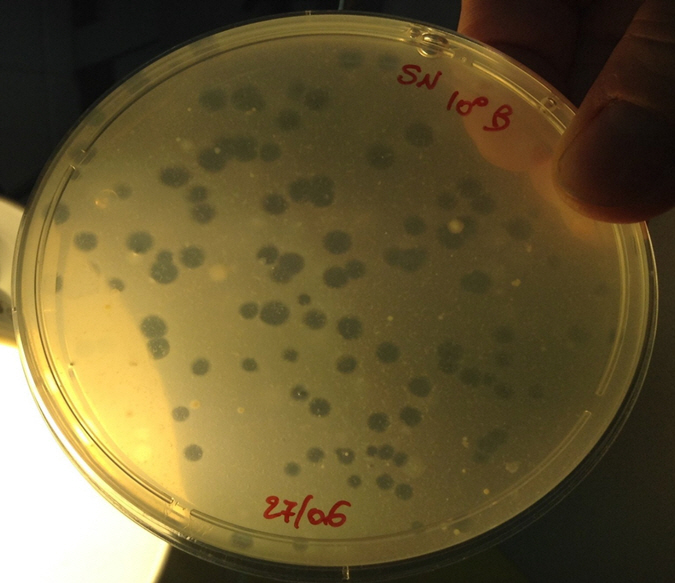
Figure 3. Indigenous BALOs isolated from our waters of
Mytilus galloprovincialis harvesting
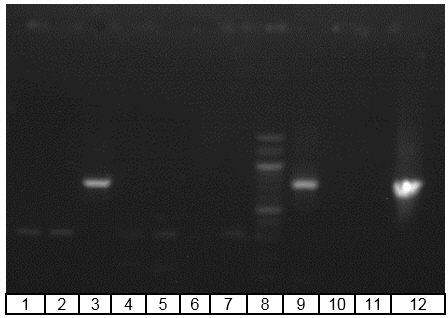
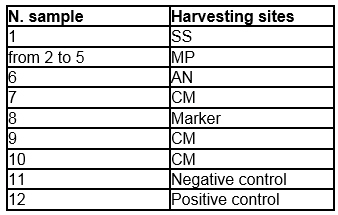
Figure 4. Gel electrophoresis of PCR analysis for identification of
Bacteriovorax spp
In parallel we are conducting studies to see if BALOs can bioaccumulate in Mytilus galloprovincialis and if they are able to eliminate V. parahaemolyticus in the experimental conditions used (Figure 5). Preliminary results suggest that BALOs are bioaccumulated from Mytilus galloprovincialis and inhibit the growth of V. parahaemolyticus. However, because in our water are naturally BALOs we must carry out further experiments to standardize the level of BALOs to be added experimentally in tests of elimination.

Figure 5. Equipment for bioaccumulation experiments with
Mytilus galloprovincialis performed at IZSUM laboratory
Our future goals will be :
1. Develop a protocol for the BALOs count from Mytilus galloprovincialis.
2. Continue to isolate and identify BALOs in our waters.
3. Standardize the optimum level of BALOs and the prey strain for use in laboratory scale tests of depuration.
4. Reproduce the experimental protocol standardized in a sewage treatment plant integrating conventional approach of depuration.
The development of effective methods for decontamination of bivalves from V. parahaemolyticus is an important goal to safeguard both the health of consumers and the production of these typical seafood. In this context, biological measures rather than chemical or physical may be perspective and our approach to implement food security linked to the consumption of these organisms.
This method could find application during the relaying process and/or in the post-harvest decontamination, substituting or integrating conventional approaches. Recently in the USA bivalves were relayed in a marine environment with a higher salinity, which was different to that of the original location of shellfish and was not favourable to target pathogens (Audemard et al, 2011). Because BALOs are tolerant to a high range of salinity, a future study could also focus on a post-harvest physical-biological integrated approach.
Moreover, being BALOs broad spectrum parasites, in addition to pathogenic bacteria they are also able to eliminate spoilage bacteria, allowing to obtain a microbiologically and organoleptically improved food. Then, purification with BALOs could be proposed as a post-harvest process for reducing vibrios, other pathogenic and spoilage bacteria present in shellfish, increase their safety and improve food quality and shelf life.
REFERENCES
Andrews LS. 2004. Strategies to control Vibrios in molluscan shellfish. Food Protection Trends 24: 70-76.
Atterbury RJ, Hobley L, Till R, Lambert C, Capeness MJ, Lerner TR. 2011. Effects of orally administered Bdellovibrio bacteriovorus on the well-being and Salmonella colonization of young chicks. Appied Environmental Microbiology. 77:5794-5803.
Barile NB, Scopa M, Nerone E, Mascilongo G, Recchi S, Cappabianca S, Antoneti L. 2009. Studio sull'efficacia di un sistema di depurazione a ciclo chiuso sui molluschi bivalvi. Veterinaria Italiana, 45: 541-553.
Cao HP, He S, Wang HC, Hou SL, Lu LQ, Yang XL. 2012. Bdellovibrios, potential bio-control bacteria against pathogenic Aeromonas hydrophila. Veterinary Microbiology. 154:413-418.
Carraturo A, Raieta K, Ottaviani D, Russo GL. 2006. Inhibition of Vibrio parahaemolyticus by a bacteriocin-like inhibitory substance (BLIS) produced by Vibrio mediterranei 1.Journal Applied Microbiology. 101: 234-241.
Croci L, Suffredini E, Cozzi L, Toti L. 2002. Effects of depuration of molluscs experimentally contaminated with Escherichia coli, Vibrio cholerae O1 and Vibrio parahaemolyticus. Journal Applied Microbiology. 92:460-465.
Dashiff A, Junka RA, Libera M, Kadouri DE. 2011. Predation of human pathogen by the predatory bacteria Micavibrio aeruginosavorus and Bdellovibrio bacteriovorus. Journal Applied Microbiology. 110:431-444.
Fratamico PM, and Cooke PH. 1996. J.Food Safety 16: 161173.
Lu F, Cai J. 2010. The protective effect of Bdellovibrio-and-like organisms (BALO) on tilapia fish fillets against Salmonella enterica ssp. enterica serovar Typhimurium. Letters in Applied Microbiology. 51:625-631.
Li H, Liu C, Chen L, Zhang X, Cai J. 2011. Biological characterization of two marine Bdellovibrio-and-like organisms isolated from Daya Bay of Shenzhen, China and their application in the elimination of Vibrio parahaemolyticus in oyster. International Journal of Food Microbiology. 151:36-43
Kadouri D and OToole GA. 2005. Susceptibility of Biofilms to Bdellovibrio bacteriovorus Attack. Applied Environmental Microbiology. 71: 4044-4051
Lee R, Lovatelli A. Ababouch L. 2008. Bivalve depuration: fundamental and practical aspects. FAO Fisheries Technical Paper No. 511.
Markelova NY. 2010. Predacious bacteria, Bdellovibrio with potential for biocontrol. International Journal Hygiene Environmental Health. 213: 428-431.
Marozzi S, Palmieri C, Narcisi F, Mosca F, Calzetta A, Malatesta D, Ottaviani D, Ghittino C, Tiscar PG. 2007. International Conference of the European Association of Fish Pathologists 267:17-22.
Mississippi Department of Marine Resources, USA, 2006. http://www.dmr.state.ms.us/Fisheries/Seafood-Technology/pdfs/fact-sheet-postharvest-oysterprocessing.pdf2006.
Ottaviani D, Leoni F, Rocchegiani E, Santarelli S, Canonico C, Masini L, DiTrani V. 2008. First Clinical Report of Pandemic Vibrio parahaemolyticus O3:K6 Infection in Italy. Journal of Clinical Microbiology. 46: 2144-2145.
Ottaviani D, Leoni F, Rocchegiani, E, Canonico C, Potenziani S, Santarelli S, Masini L, Mioni R, Carraturo A. 2010. Vibrio parahaemolyticus in mussels from Italian growing areas, Adriatic Sea. Environmental Microbiology Reports 2:192-197.
Ottaviani D, Leoni F, Rocchegiani E, Canonico C, Potenziani S, Santarelli S, Masini L, Scuota S, Carraturo A. 2010. Vibrio parahaemolyticus-associated gastroenteritis in Italy: persistent occurrence of O3:K6 pandemic clone and emergence of O1:KUT serotype. Diagnostic Microbiology and Infectious Disease. 66: 452-455.
Ottaviani, F. Leoni, E. Rocchegiani, R. Mioni, A. Costa, S. Virgilio, L. Serracca, D. Bove, C. Canonico, A. Di Cesare, L. Masini, S. Potenziani, G. Caburlotto, M. M. Lleo. 2013. An extensive investigation into the prevalence and the genetic and serological diversity of toxigenic Vibrio parahaemolyticus in Italian marine coastal water. Environmental Microbiology. 15: 1377-1386.
Ottaviani D, Leoni F, Serra R, Serracca L, Decastelli L, Rocchegiani E, Masini L, Canonico C, Talevi G, Carraturo A. 2012. Nontoxigenic Vibrio parahaemolyticus Strains Causing Acute Gastroenteritis. Journal of Clinical Microbiology 50: 4141-4143.
Potasman I, Paz A, and Odeh M. 2002. Infectious outbreaks associated with bivalve shellfish consumption: a worldwide perspective. Clinical Infectious Disease 35:921-928.
Richards GP, Fay JP, Dickens KA, Parent MA, Soroka DS, Boyd EF. 2012. Predatory bacteria as natural modulators of Vibrio parahaemolyticus and Vibrio vulnificus in seawater and oysters. Applied of Environmental Microbiology, 78, 7455-7466.
Ruby E G. The genus Bdellovibrio. In: Balows, A.,H. G. Truper, M. Dworkin, W. Harder, and K. H. Schleifer (eds), The Prokaryotes, 2nd edn, 1991. pp. 34003415. Springer Verlag, New York, NY.
Stolp H and Starr M P. 1963. Bdellovibrio bacteriovorus gen. et sp. n., a predatory, ectoparasitic, and bacteriolytic microorganism. Antonie Van Leeuwenhoek 29: 217-248.
Wagner M and Loy A. 2002. Bacterial community composition and function in sewage treatment systems. Current Opinion in Biotechnology. 13: 218-227.









